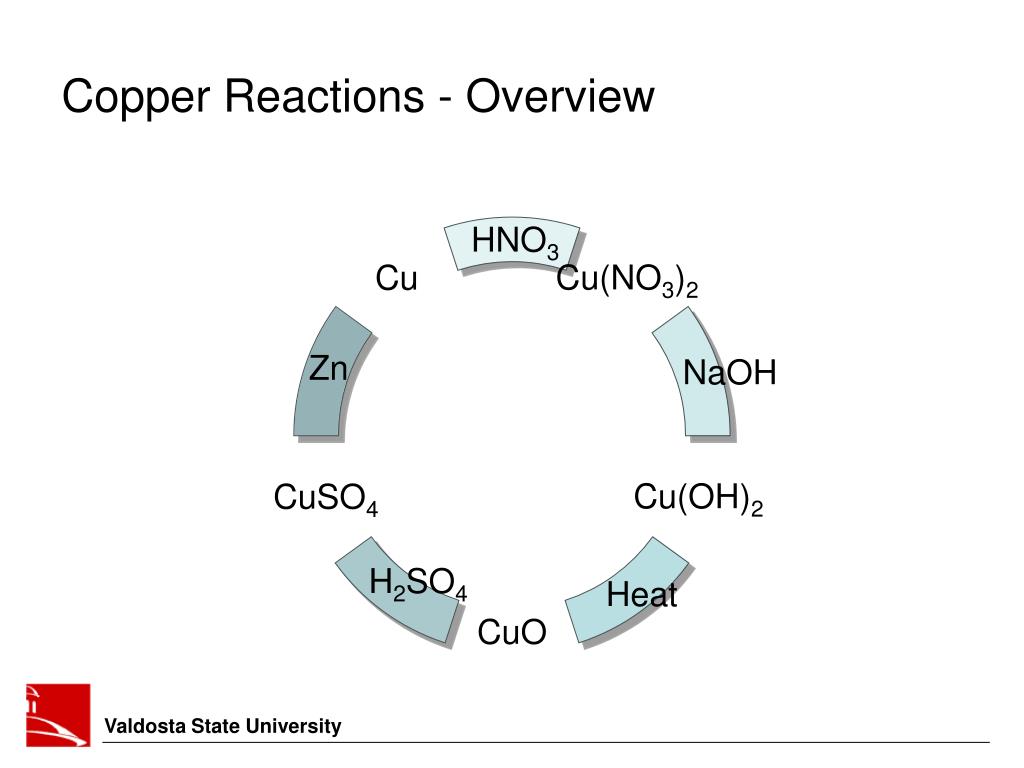
Copper Hydroxide Reaction Products . copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Hydroxide ions (from, say, sodium. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. reactions of hexaaquacopper(ii) ions with hydroxide ions. It can be converted to other copper compounds by acidification. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. heating copper hydroxide produces copper oxide, cuo, a black solid. copper (ii) hydroxide is an reactive hydroxide of copper. To learn more about the structure,. the two ions combine together to form an insoluble salt. Cu (oh) 2 + hx → cux + 2. Copper oxide dissolves in acid, regenerating the copper.
from www.slideserve.com
reactions of hexaaquacopper(ii) ions with hydroxide ions. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Cu (oh) 2 + hx → cux + 2. copper (ii) hydroxide is an reactive hydroxide of copper. copper hydroxide reacts with sulfuric acid to form copper sulfate and water. To learn more about the structure,. Copper oxide dissolves in acid, regenerating the copper. heating copper hydroxide produces copper oxide, cuo, a black solid. Hydroxide ions (from, say, sodium. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2.
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation
Copper Hydroxide Reaction Products the two ions combine together to form an insoluble salt. Copper oxide dissolves in acid, regenerating the copper. It can be converted to other copper compounds by acidification. Cu (oh) 2 + hx → cux + 2. heating copper hydroxide produces copper oxide, cuo, a black solid. copper (ii) hydroxide is an reactive hydroxide of copper. copper hydroxide reacts with sulfuric acid to form copper sulfate and water. To learn more about the structure,. the two ions combine together to form an insoluble salt. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Hydroxide ions (from, say, sodium. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. reactions of hexaaquacopper(ii) ions with hydroxide ions.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Hydroxide Reaction Products the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Hydroxide ions (from, say, sodium. copper (ii) hydroxide is an reactive hydroxide of copper. It can be converted to other copper compounds by acidification. Cu (oh) 2 + hx → cux + 2. reactions of hexaaquacopper(ii) ions with hydroxide. Copper Hydroxide Reaction Products.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube Copper Hydroxide Reaction Products copper (ii) hydroxide is an reactive hydroxide of copper. copper hydroxide reacts with sulfuric acid to form copper sulfate and water. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Hydroxide ions (from, say, sodium. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with. Copper Hydroxide Reaction Products.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Copper Hydroxide Reaction Products Cu (oh) 2 + hx → cux + 2. copper hydroxide reacts with sulfuric acid to form copper sulfate and water. It can be converted to other copper compounds by acidification. To learn more about the structure,. the two ions combine together to form an insoluble salt. Copper oxide dissolves in acid, regenerating the copper. the production. Copper Hydroxide Reaction Products.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper Hydroxide Reaction Products It can be converted to other copper compounds by acidification. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. copper (ii) hydroxide is an reactive hydroxide of copper. To learn more about the structure,. the two ions combine together to form an insoluble salt. Copper oxide dissolves in. Copper Hydroxide Reaction Products.
From wisc.pb.unizin.org
4.2 Classifying Chemical Reactions Chemistry Copper Hydroxide Reaction Products the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. It can be converted to other copper compounds by acidification. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. To learn more about the structure,. Cu (oh) 2 + hx → cux + 2. Hydroxide. Copper Hydroxide Reaction Products.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II Copper Hydroxide Reaction Products To learn more about the structure,. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. heating copper hydroxide produces copper oxide, cuo, a black solid. Cu (oh) 2 + hx → cux + 2.. Copper Hydroxide Reaction Products.
From www.youtube.com
Reaction between copper(II)sulfate and potassium hydroxide YouTube Copper Hydroxide Reaction Products copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Copper oxide dissolves in acid, regenerating the copper. Cu (oh) 2 + hx → cux + 2. copper hydroxide reacts with sulfuric acid to form copper sulfate and water. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii). Copper Hydroxide Reaction Products.
From fineartamerica.com
Copper Hydroxide Precipitate Photograph by Andrew Lambert Photography Copper Hydroxide Reaction Products copper (ii) hydroxide is an reactive hydroxide of copper. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. reactions of hexaaquacopper(ii) ions with hydroxide ions. Hydroxide ions (from, say, sodium. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. Copper oxide dissolves. Copper Hydroxide Reaction Products.
From www.youtube.com
Amino Acids 3. Reactions with sodium hydroxide, sodium carbonate Copper Hydroxide Reaction Products the two ions combine together to form an insoluble salt. Cu (oh) 2 + hx → cux + 2. copper hydroxide reacts with sulfuric acid to form copper sulfate and water. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. Hydroxide ions (from, say, sodium. copper(ii) ion. Copper Hydroxide Reaction Products.
From www.grosafe.co.nz
Hortcare Copper Hydroxide 10kg Grosafe Copper Hydroxide Reaction Products heating copper hydroxide produces copper oxide, cuo, a black solid. reactions of hexaaquacopper(ii) ions with hydroxide ions. copper (ii) hydroxide is an reactive hydroxide of copper. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts. Copper Hydroxide Reaction Products.
From www.canfuo.com
Copper Hydroxide NanorodsNano Products Copper Hydroxide Reaction Products copper (ii) hydroxide is an reactive hydroxide of copper. reactions of hexaaquacopper(ii) ions with hydroxide ions. It can be converted to other copper compounds by acidification. To learn more about the structure,. Cu (oh) 2 + hx → cux + 2. heating copper hydroxide produces copper oxide, cuo, a black solid. the two ions combine together. Copper Hydroxide Reaction Products.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper Hydroxide Reaction Products copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. heating copper hydroxide produces copper oxide, cuo, a black solid. the two ions combine together to form an insoluble salt. copper hydroxide reacts with sulfuric acid to form copper sulfate and water. reactions of hexaaquacopper(ii) ions with hydroxide ions. To learn. Copper Hydroxide Reaction Products.
From slideplayer.com
Types of Reactions. ppt download Copper Hydroxide Reaction Products Cu (oh) 2 + hx → cux + 2. heating copper hydroxide produces copper oxide, cuo, a black solid. reactions of hexaaquacopper(ii) ions with hydroxide ions. It can be converted to other copper compounds by acidification. copper hydroxide reacts with sulfuric acid to form copper sulfate and water. Hydroxide ions (from, say, sodium. the two ions. Copper Hydroxide Reaction Products.
From www.sciencephoto.com
Copper Hydroxide Precipitate Stock Image C027/9441 Science Photo Copper Hydroxide Reaction Products the two ions combine together to form an insoluble salt. reactions of hexaaquacopper(ii) ions with hydroxide ions. To learn more about the structure,. Hydroxide ions (from, say, sodium. copper(ii) ion reacts with stoichiometric quantities of aqueous ammonia to precipitate light blue cu(oh)2. copper hydroxide reacts with sulfuric acid to form copper sulfate and water. the. Copper Hydroxide Reaction Products.
From www.youtube.com
Making Copper Hydroxide YouTube Copper Hydroxide Reaction Products Hydroxide ions (from, say, sodium. To learn more about the structure,. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. heating copper hydroxide produces copper oxide, cuo, a black solid. the two ions combine together to form an insoluble salt. reactions of hexaaquacopper(ii) ions with hydroxide ions.. Copper Hydroxide Reaction Products.
From www.youtube.com
Copper (ii) Acetate to Copper Hydroxide reaction. YouTube Copper Hydroxide Reaction Products the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. heating copper hydroxide produces copper oxide, cuo, a black solid. reactions of hexaaquacopper(ii) ions with hydroxide ions. It can be converted to other copper compounds by acidification. copper (ii) hydroxide is an reactive hydroxide of copper. To learn. Copper Hydroxide Reaction Products.
From www.pinterest.com
3 tube tests containing 1) CuSO4 copper sulphate VI 2) Cu(OH)2 Copper Hydroxide Reaction Products It can be converted to other copper compounds by acidification. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. To learn more about the structure,. Cu (oh) 2 + hx → cux + 2. the two ions combine together to form an insoluble salt. copper (ii) hydroxide is. Copper Hydroxide Reaction Products.
From apparentag.com.au
Apparent Copper Hydroxide Apparent Ag Copper Hydroxide Reaction Products copper hydroxide reacts with sulfuric acid to form copper sulfate and water. the production of copper (ii) hydroxide typically occurs through the reaction of copper (ii) salts with an alkali. It can be converted to other copper compounds by acidification. Cu (oh) 2 + hx → cux + 2. the two ions combine together to form an. Copper Hydroxide Reaction Products.